Nursing News and Information
The CPSC (US Consumer Product Safety Commission), in cooperation with Sprout Stuff of Austin, Texas; has recalled 40 Sprout Stuff infant ring slings. The CPSC urges consumers to discontinue use of these slings since they pose a risk of suffocation to infants. A 10 day old boy in Texas died due to the use of this sling. Consumers can get a full refund for these slings from the company. They sold for about $35 to $45 from October 2006 to May 2007 and were sold directly from the manufacturer. The company is working to contact all purchasers. This recall follows on the heels of other major recalls for baby sling carriers. The CPSC previously recalled 1,200 Ellaroo Ring Sling baby carriers, 165 ZoloWear Infant Carriers/Slings, and approximately 100,000 Infantino SlingRider infant carriers.
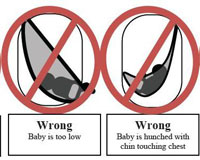
In general, infant baby slings present two suffocation dangers. Since babies cannot control their heads in the first few months of life due to weak musculature, the sling can press against the nose and mouth. This can lead to suffocation within one to two minutes. Also, the sling may rest the baby in a curled position with the chin bending toward the chest. In this scenario, airways are restricted and the baby cannot cry for help while slowly suffocating.
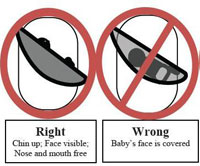
The CPSC has also noted that improper use of even the best sling carriers for infants is dangerous. The US CPSC provides important instructions on proper use. The CPSC “recommends that parents and caregivers make sure the infant’s face is not covered and is visible at all times to the sling’s wearer. If nursing the baby (is) in a sling, change the baby’s position after feeding so the baby’s head is facing up and is clear of the sling and the mother’s body.” Frequent checking of the baby’s position is urged, especially after nursing. “CPSC has determined that a mandatory standard is needed for infant sling carriers. While a mandatory standard is being developed, CPSC staff is working with ASTM International to quickly develop an effective voluntary standard for slings. There currently are no safety standards for infant sling carriers.”
Below is a photo of the Sprout Stuff sling that has been recalled and the other diagrams demonstrate proper and improper ways to use a baby sling. The diagrams are provided by the US Consumer Product Safety Commission.


The US Consumer Product Safety Commission, in cooperation with Sony Electronics Inc. of San Diego, California; has recalled 233,000 Sony VAIO laptop computers due to the possibility of burn hazards. The computers may overheat and reports of units overheating resulting in deformed keyboards and casings prompted the recall. The overheating problem is caused by a malfunction of the computer’s internal temperature management system. These computers were sold from January 2010 to April 2010 at places such as: Best Buy, Sony Style, Costco, Frys, and Amazon.com . They came in many colors and are part of the VPCF11 and VPCCW2 series of notebook computers. Owners can check with Sony to see if their computer is part of the recall at telephone number 866-496-7669. These computers were manufactured in China and the United States, have VAIO clearly marked on the outside panel, and it is illegal to resell the affected units. The US CPSC urges consumers to discontinue using these units and to perform an update on the BIOS firmware to remedy this hazard. Consumers can call Sony or visit a Sony Style retail store for help with installing the update. This firmware update fixes the overheating problem in all of the affected computers. To date, no injuries have been reported. However, both Sony and the US CPSC warm against using these computers until the firmware update has been performed.

Blue Cross Blue Shield of Michigan (BCBSM) has asked permission for a rate hike of up to 15% for nearly 200,000 individual subscribers. Hikes for group subscribers will range from 8-12%. The premium increase must be approved by the Michigan Office of Financial and Insurance Regulation for it to take effect. BCBSM claims that they will lose over $50 million dollars without the hike.
This may come as a surprise to many policy holders since Michigan’s insurance commissioner approved a 22% increase last year for both individual and group policy holders. Despite the increase, BCBSM claims to have lost nearly $100 million last year. Michigan Attorney General Mike Cox has questioned the loss claims since BCBSM increased its surplus by $528 million in 2009. That is profit on top of the already $2 billion surplus that the company holds. Cox has also questioned the purported losses because the State of Michigan gives the company $100 million in tax breaks every year to help control costs and prevent losses.
Last year, Attorney General Cox asked Blue Cross Blue Shield of Michigan pointed questions about their finances. In a letter to BCBSM, Cox asked about bonuses and salaries to board members, perks such as entertainment expenses, and the total value of the BCBSM art collection. Cox also asked for BCBSM to state the amount of funds transferred to affiliated companies. The Attorney General estimated the transfer of funds out of BCBSM to be over $450 million since 2005. Such a transfer would then show up as a loss for the company even though the funds are received by affiliates. This line of inquiry shows that the Michigan Attorney General suspects clever accounting techniques and financial manipulation to help show a loss for BCBSM. At the same time, Michigan Congressmen John D. Dingell and Sander Levin questioned Blue Cross Blue Shield of Michigan President and CEO Daniel J. Loepp about proposed rate hikes. The Congressmen asked questions concerning how much of the rate hikes will go towards paying benefits to subscribers, why the BCBSM massive surplus is not used to offset any losses, and what types of bonuses did employees making more than $500,000 per year receive. This line of inquiring was partially triggered by BCBSM paying enormous bonuses to executives prior to announcing a reduction of its workforce, salary freezes, and rate hikes. Blue Cross Blue Shield of Michigan CEO Leopp had a 23% increase in salary in 2009 to $1.12 million. However, his bonus pay has now dropped to approximately $650,000 down from last year’s $888,269.

The FDA has just approved a new treatment for advanced prostate cancer. The FDA has approved Provenge, a cellular immunotherapy drug. It is the first drug approved by the FDA in its class. Provenge works by stimulating the body’s own immune system to attack the cancer. Skin cancer is the most common form of cancer amoung men in the U.S and prostate cancer is the second most common form. The National Cancer Institute reports that in 2009 approximately 27,000 men died from prostate cancer and 192,000 new cases were reported.
Provenge is manufactured using the patient’s blood. Immune cells from the blood sample are processed to enhance their ability to fight cancer in a process known as leukapheresis. The cells are then re-administered to the patient intravenously in three doses. The FDA was prompted to approve this therapy following a study of 512 patients with advanced prostate cancer. Patients taking Provenge lived an average of 4 months longer than those who did not receive the treatment. Nearly every patient who received the Provenge therapy experienced adverse reactions including back pain, joint pain, chills, fatigue, fever, headache, nausea, or stroke. Provenge costs $93,000 for a total of three required dosages.

President Obama has nominated Donald Berwick to run Medicare and Medicaid. Dr. Berwick, M.D., M.P.P. is a Professor of Health Policy and Management at the Harvard School of Public Health, a pediatrician, President of the Institute for Healthcare Improvement (IHI), and a consultant in pediatrics to Massachusetts General Hospital. He is widely published on the topic of health care systems with a focus on improving access to quality medical care. His books include Curing Health Care, New Rules: Regulation, and Markets and the Quality of American Health Care. His scientific articles have been published in The Journal of the American Medical Association, The New England Journal of Medicine, The British Medical Journal, and many other prestigious medical periodicles. President Clinton appointed Dr. Berwick to serve on the Advisory Commission on Consumer Protection and Quality in the Healthcare Industry. He received his MD from Harvard Medical School and his MPP from the John F. Kennedy School of Government.
Dr. Berwick is controversial for the statement, "...any health care funding plan that is just, equitable, civilized and humane must, must redistribute wealth from the richer among us to the poorer and the less fortunate. Excellent health care is, by definition, redistributional." Central medical ideas to the IHI and Dr. Berwick are no needless deaths, pain, or suffering; no waiting, no waste, and access to medical care for all. He has numerous awards and honors for his achievements in healthcare including the Heinz Award for Public Policy, the Award of Honor from the American Hospital Association, and the Alfred I. DuPont Award. Many of Dr. Berwicks’s achievements seek to not only improve the quality of medical care but also to improve the safety of the healthcare setting for both patients and healthcare workers.

Seasonal flu vaccines have been linked to increased outbreaks of H1N1 (Swine Flu) in four different epidemiological studies. Serious questions have been raised concerning the biological interactions between influenza strains and vaccines. Patients who received the seasonal “flu shot” in 2008 and 2009 had a significant increase of developing H1N1. Both the seasonal flu and the pandemic H1N1 flu are due to strains of influenza virus and separate vaccinations are used to prevent each illness. These findings may impact recommendations by doctors and nurses for patients seeking medical information.
An initial inquiry began after investigators noticed that the majority of people with illnesses characterized by fever and coughing had received the seasonal flu vaccine. The investigation was initiated to explain this phenomenon and looked at people receiving seasonal flu shots (TIV) and their subsequent outbreaks of H1N1. Four separate epidemiological studies were launched to study the cause and means of this phenomenon. The studies revealed that TIV vaccines were effective in reducing seasonal flu outbreaks but increased a person’s chances of getting the swine flu (H1N1). All four studies showed that those who received the seasonal flu vaccine had approximately twice the chance of developing the H1N1 swine flu. The study included researchers from the British Columbia Center for Disease Control (BCCDC) in Vancouver, the National Microbiology Laboratory in Winnipeg, Canada; and the Ontario Agency for Health Protection and Promotion.
A study published in PLoS medicine in April 2010 revealed the unexpected findings from the four epidemiological findings. The trivalent inactivated influenza vaccine (TIV) was shown to have a counterproductive effect toward H1N1. Patients presenting with influenza syndromes were tested by a network of physicians for influenza virus with RT-PCR and an investigation of demographics, clinical outcomes, and vaccine status. Influenza-positive patients were then compared with influenza-negative patients. The results confirmed that TIV lowered the incidence of seasonal flu outbreaks when properly matched with circulating influenza strains in a given season. Alternately, mismatched vaccines did not have the same clinical benefits. However, even with adjustments for comorbidities, age, and geography; the seasonal flu vaccine (TIV) appeared as a risk factor for the swine flu for those under 50 years of age. The four studies conclude that recipients of the seasonal flu vaccine are approximately twice as likely to get the swine flu (H1N1). A causal link has not been established between TIV and H1N1 and investigators suggest further studies to explain this unexpected phenomenon. Both nurses and doctors will be better informed regarding patient recommendations once further study has been completed.
The US Consumer Product Safety Commission (CPSC) has announced a voluntary recall of baby cribs which pose strangulation and suffocation hazards. Approximately 635,000 baby cribs made by Dorel Asia have been recalled because the drop side hardware can fail and trap the infant in the opening between the drop side and the crib. A six month old child from Cedar Rapids, Iowa was entrapped and died due to strangulation thereby prompting this recall. Several other incidents were reported in which children received bruises and scratches as a result of entrapment. These cribs were sold at K-Mart, Wal-Mart, and Sears from January 2005 to December 2009 and sold for between $120 and $700. The CPSC has issued a warning to parents and caregivers. Tighten the hardware occasionally to make sure the crib is sturdy. Also, check any moving parts, especially drop sides, to ensure that they operate smoothly. The CPSC notes to check sides and corners such that there are no gaps that may entrap a child. Also, the CPSC strongly warns not to repair the sides of the crib, especially with duct tape, wire, or rope. In the past, other major recalls for cribs were issued by the CPSC including 985,000 Delta Enterprise drop side cribs. Also, 2.1 million Stork Craft drop side cribs, some under the Fisher-Price logo, have been recalled. Several issues have been noted such as broken claw attachment pieces and upside down installation of the drop side. The manufacturer will issue a repair kit for these cribs and users are urged to stop using these units. Stork Craft cribs are distributed in the USA and Canada and were sold at major retailers such as BJ’s Wholesale Club, J.C. Penney, K-mart, Sears, USA Baby, and Wal-Mart stores and online at Amazon.com, Babiesrus.com, Costco.com, Target.com, and Walmart.com . Many of these cribs have the terms storkcraft baby and storkling on the drop side teething rail.
It is important to look for little gaps in which infants can get stuck by performing a careful inspection of the hardware. Importantly, children can slide in between the mattress area and the movable drop side of the crib. Make sure the children cannot slip into this area. This helps ensure safety for the child and peace of mind for caregivers and parents. The role of the CPSC is to oversee hazard identification, safety standards, compliance and enforcement, public outreach, and intergovernmental cooperation relating to consumer product-related injuries. The crib recall is voluntary with cooperation of the manufacturer. The manufacturer will issue fixes and consumers are urged to stop using the recalled cribs until replacement kits are installed. Contact the CPSC for further information to assist you with the recall.

Under direction of the Obama administration, the EPA (US Environmental Protection Agency) and the DOT (Department of Transportation) have set new federal emission standards for passenger cars and light trucks. National greenhouse gas emissions have been established and tighter fuel economy requirements will affect 2012 through 2016 model-year vehicles. The EPA estimates that the improved mileage could save consumers up to $3,000 over the life of a vehicle. In balance, the Obama administration has released these new ecologically oriented standards on the heels of announcing the opening up of massive amounts of US terrain to offshore oil and gas exploration.
The Transportation Secretary states that Americans will “save money at the pump while putting less pollution in the air” because of the new regulations. EPA administrator Lisa Jackson noted that this move combines economic opportunities with “environmental priorities.” In a new effort of collaboration and simplification, cars and light trucks will meet only one federal standard instead of having to meet three separate standards set by the State, DOT, and EPA.
By 2016, a manufacturer’s fleet must average 34.1 mpg. This is achieved by requiring a 5% per year increase in fuel efficiency leading up to the 2016 standard. For cars and light trucks produced from 2012 to 2016 this is a reduction of 960 million metric tons of carbon dioxide emissions over the lifetime of the vehicles. The EPA notes this is the equivalent of taking 50 million cars and light trucks off the road in 2030. This also saves 1.8 billion barrels of oil. The Obama administration worked towards these cleaner air standards and increased fuel efficiency standards in a joint effort with automakers, the United Auto Workers, States, and many involved in the environmental community.
These standards make substantial gains in air quality and may help prevent toxic brown clouds from appearing over cities while reducing dependency on oil. This may help to reduce respiratory illnesses in populations due to air pollution. While these new emission standards are cleaner than they have ever been, they pale in comparison to zero emissions vehicles (ZEVs) which are already on American roads. ZEVs are vehicles that emit no tailpipe pollutants. This includes electric vehicles and vehicles powered by hydrogen fuel cells. Hydrogen fuel cell vehicles emit only water. It is safe to drink the water from the tailpipe! Rather than convert the entire fleet to zero emissions, the Obama administration has taken a moderate approach by requiring a slow shift to higher efficiency vehicles.
Multivitamin use is linked to an increased rate of breast cancer according to newly released information from the Division of Nutritional Epidemiology in Stockholm Sweden. A statistically significant increase in breast cancer was discovered in this multiyear study with a sample size of 35,329 participants. This adds to increasing concern over the safe use of vitamins. Prior, the National Cancer Institute (a division of the U.S. National Institute’s of Health) also concluded that beta-carotene supplements increase the risk of lung cancer. The National Cancer Institute reports that “contrary to earlier expectations, not only do beta-carotene supplements not prevent lung cancer in people at high risk for the disease, they appear to increase rates of the disease, particularly among smokers.” In another study, Dr. Slatore, MD of the University of Washington in Seattle notes that “increased intake of supplemental vitamin E was associated with a slightly increased risk of lung cancer." This conclusion was reached from a study with a sample size of 77,000 participants and was published in the American Journal of Respiratory and Critical Care Medicine. On the other hand, many studies link healthy levels of vitamin D to significantly lowering the risk for breast, colon, and pancreatic cancer.
Several factors are involved in healthy and safe vitamin supplementation. Concentration levels vary. Some vitamins offer extremely high dosages of particular nutrients with a ‘more is better’ approach. However, an excess of nutrients may be as dangerous as a deficiency. For example, iron supplements are helpful for those suffering from iron deficiency anemia but are toxic and perhaps deadly to those with a rare disorder known as hemochromatosis. Therefore, appropriate supplementation is dependent on the need for a vitamin. If someone already has healthy levels of a vitamin, adding more can be detrimental.
Manufacturing quality varies amongst vitamin producers. The FDA’s GRAS (Generally Recognized As Safe) category allows companies to add substances, including newly developed chemicals, into vitamins without the FDA’s approval or knowledge. Companies are not required to identify substances added to vitamins such that neither the consumer nor the FDA knows what ingredients are contained within vitamin supplements. Companies may add engineered nanomaterials (materials manipulated at the molecular level) and other substances into vitamins without notifying the FDA. Companies may make their own determination of whether or not a vitamin is GRAS and companies are not required to tell the FDA if the substance exists in their products or how the GRAS determination was made. A U.S. GAO (General Accountability Office) report states, “FDA does not know to what extent, or even whether, companies track evolving scientific information about their GRAS substances. FDA's approach to regulating nanotechnology allows engineered nanomaterials to enter the food supply as GRAS substances without FDA's knowledge.” Therefore, not all vitamins are equal and quality matters. Essentially, consumers need to know what undeclared substances are added to their vitamins, however, there are no labeling requirements to help consumers make that determination. Some vitamins are made from primarily organic food sources such as in the Standard Process brand. This company concentrates certified organic foods into vitamin pills thereby setting a high standard of safety for the industry. This is a professional brand and is available through doctors, nurses, acupuncturists, and other licensed medical professionals.


