Nursing News and Information
The United States Department of Labor’s Bureau of Labor Statistics has released its 2010-11 outlook for the nursing profession. The department notes that registered nurses (RNs) constitute the largest healthcare occupation with a total of 2.6 million jobs in 2008. Approximately 60% of all nurses work in hospitals. Another 8% of jobs were in physician offices, 5% in home healthcare, 5% in nursing care facilities, and 3% in employment services. Job opportunities are rated as excellent regarding growth in the demand for nurses in the healthcare industry. Many employers are reporting shortfalls of nursing staff and are having difficulties attracting nurses to their facilities.
Median wages for RNs were approximately $62,500 in 2008. The highest 10% of nurses earned an average of approximately $92,500. Nursing remains attractive because many employers offer flexible work schedules, child care, educational benefits, and bonus programs. 21% of nurses are union members. Looking to the future, an increase in educational opportunities for students to train in the nursing profession is essential to meet upcoming demand. Lack of available space for qualified applicants at nursing schools persists and is fueling an increasing shortage of filled nursing positions.
The numbers are staggering. In 2008 there were 2,618,700 nurses in the US. It is projected that there will be a need for 3,200,200 by 2018. That's a creation of 581,500 new nursing positions, a 22% increase. However, the rate of employment growth is not projected to be even across all nursing healthcare settings. Growth of nursing jobs in hospitals is expected to grow only 17% while growth in physician’s offices will grow at a 48% rate. This is attributed to the slow growth of inpatients (patients remaining in the hospital over 24 hours), earlier discharges, and an increase in outpatient procedures. Home healthcare services will grow at a rate of 33%, nursing care facilities at a 25% rate, and RN jobs in employment services at a 24% rate. An aging population combined with financial pressures on hospitals to release patients earlier will drive the growth rate of residential care facilities and home healthcare. A spike in job growth is anticipated for nurses providing care for the long-term rehabilitation of stroke and head injury patients and also patients suffering from Alzheimer’s disease.
Nursing education is continuing to run into difficulties. A continuing increase in nursing education applicants has caused many nursing schools to reject qualified applicants due to a shortage of nursing faculty. An increase in availability in nursing schools and nursing continuing education programs could help meet the growing demand. Hospitals will also face difficulties in attracting nurses to their facilities. Although the growth in new nursing jobs at hospitals is expected to be only 17%, the high turnover rate of existing jobs will drive shortages. The largest shortages are expected in rural areas and inner city regions. An especially high demand is expected for advanced practice specialties in nursing: clinical nurse specialists, nurse practitioners, nurse-midwives, and nurse anesthetists. In many areas, nurses will represent a substantial percentage of primary care providers to the public.

President Barack Obama has released a memorandum outlining the importance of combating childhood obesity. In it, the President outlines strategies to address weight issues in the nation’s youth. Public outreach and the formation of a task force are central to this initiative. First Lady Michelle Obama takes a lead role in bringing national awareness concerning this epidemic through the Let’s Move organization. She works with public, nonprofit, and private sectors to support measures to address childhood obesity. Let’s Move also focuses on working with families and communities.
Approximately 30% of children in the USA are overweight. This rate has doubled in young children and has tripled for adolescents since 1980. It is estimated that at least one third of today’s youth will suffer from diabetes and others will contract other obesity related illnesses including high blood pressure and heart disease. The presidential directive states that immediate action is required. As such, the directive focuses on preventing obesity related illnesses and consequential burdens on the nation’s healthcare system.
The memorandum establishes a task force which focuses on parent nutritional education, physical exercise, and affordable food options in schools and communities. The task force includes cabinet members such as the Secretaries of the Interior, Agriculture, Health and Human Services, and Education. The Assistant to the President and the Chief of Staff to the First Lady are also included in the task force. The task force will focus on federal responses to the obesity crisis as well as nongovernmental actions that can be implemented. Special attention will be given to healthy, affordable food; increased physical activity in schools and communities, healthier food in schools, and informing parents and caregivers concerning healthy choices. Let’s Move takes up these initiatives and actively works to achieve these health related goals.
Building on this momentum, the new health reform law requires that chain restaurants and vending machines with more than 20 locations to label food calorie information. The new health reform law, the Patient Protection and Affordable Care Act, signed recently by President Obama provides funding for a childhood obesity reduction project. The health reform law identifies obesity reduction as an important method to prevent illness. As such, obesity related services take an important role in the Patient Protection and Affordable Care Act and obesity related healthcare is cited multiple times in the 2,393 page law. Educational and counseling services as well as community funding for projects to end obesity are included in the new law.
President Bill Clinton’s foundation, in conjunction with the American Heart Association, created the Alliance for a Healthier Generation which shares the same goals as the Obama initiative. Partnership for a Healthier America is a foundation newly formed to combine multiple nonpartisan efforts to fight obesity. This new foundation combines efforts with the Alliance for a Healthier Generation, The California Endowment, Kaiser Permanente, Nemours, the Robert Wood Johnson Foundation, and the W.K. Kellogg Foundation. First Lady Michelle Obama serves as Honorary Chair and both a prominent Republican and a prominent Democrat will be named as Honorary Vice Chairs to underscore the organization’s nonpartisan position.
The bipartisan and nonpartisan fitness initiatives and organizations of today starkly contrast the USDA’s (US Department of Agriculture) reclassification of ketchup as a vegetable during President Reagan’s term in office. This allowed school lunch programs to meet minimum standards required for federal reimbursements even if they replaced vegetables with a small amount of ketchup. Overnight, vegetables disappeared from school lunch programs and paper containers with a small helping of ketchup appeared on school cafeteria trays. Democratic politicians seized the opportunity to dramatize this USDA snafu, termed ketchupgate, by staging photo ops of democrats dining on nutritionally void meals that met the new lax standards. Newsweek magazine reported the story and the ketchup as a vegetable policy was subsequently abandoned.

The FDA notes that there is no evidence of a safety concern from the contaminated Rotarix vaccine. The suspension of its use is viewed as a preventative measure. The FDA will conduct research into the viral contamination issue to determine whether the PCV1 contaminant is an intact virus or merely DNA fragments. Additional recommendations concerning the vaccine will be made in about one to two months when the FDA is convening an expert panel to look into this issue. The FDA will report this information to patients, doctors, nurses, and the World Health Organization (WHO) as information surfaces.
The Director of the Centers for Disease Control and Prevention speculates that many countries will continue to use the Rotarix vaccine until further information becomes available. Citing dangers of rotavirus, the director anticipates that the vaccine warning will be weighed against the threat of the disease. In balance, he feels that many countries will risk the potential dangers of the vaccine to prevent rotavirus outbreaks. RotaTeq vaccine, manufactured by Merk pharmaceuticals, is not affected by this FDA warning. RotaTeq is also used to prevent rotavirus and no viral contamination has been discovered in this product.
The EPA (US Environmental Protection Agency) is tightening controls over flea and tick “spot-on” pesticides for pets to prevent adverse reactions. Spot-on pesticides are liquids that are usually squeezed onto a dog or cat’s skin between the shoulder blades or on the back. The EPA is moving to require improved labeling to ensure that cats do not receive spot-on flea and tick pesticides intended for dogs and that smaller dogs do not receive spot-on dosages that are only appropriate for larger, heavier dogs. The EPA notes that using the improper type of spot-on product or an excessive dosage may lead to skin disorders, digestion issues, vomiting, diarrhea, and nervous system disorders such as trembling and seizures. Also at risk are pets that are weak, old, medicated, ill, pregnant or are nursing. In these circumstances, the FDA notes that pet owners are advised to consult with veterinarians concerning the application of flea and tick pesticides.
The EPA states that “flea and tick products can be appropriate treatments for protecting pets and public health because fleas and ticks can transmit disease to animals and humans.” Dogs and cats exposed to fleas may suffer from hair loss, skin disorders such as dermatitis, anemia, and in some cases the pets may become seriously ill. Ticks may transmit serious bacterial infections such as Lyme’s disease to pets. Pets may also shed ticks in the home potentially exposing humans to Lyme’s disease and other dangerous infectious diseases such as Ehrlichiosis and Bebesia. Transmitted by tick bites, these infections require immediate medical attention to prevent potentially life-threatening illness. In balance, the EPA supports the use of flea and tick pesticides to prevent disease while urging pet owners to pay careful attention to their proper application in order to prevent adverse side-effects to animals.
In the majority of cases, adverse effects do not occur as a result of using spot-on products. However, the EPA’s analysis finds that improvements in packaging and labeling will significantly lower the risk of adverse side effects. Smaller dog breeds are at risk because they often receive dosages intended for larger dogs. In many cases, the EPA notes that consumers are applying spot-on dog products to cats thereby increasing the probability of adverse effects. The EPA adds that children are protected from exposure to spot-on pesticide treated pets based on dermal assessment reviews of exposures due to hugging and coming into contact with pesticide treated pets. Inhalation of spot-on pesticides remains a case-by-case exposure issue.

A recent study at the University of California, Los Angeles, reveals that 8.2 million Californians are without health insurance. This is a 28% increase from the 6.4 million uninsured Californians in 2007. The UCLA Center for Health Policy Research study notes that approximately 2 million Californians lost health insurance coverage from 2008 to 2009 bringing the total to over 8 million uninsured. Currently, 25% of all California residents do not have health insurance. The study cites rising unemployment rates and health insurance premium increases as major contributing factors to this problem. The study was funded by the California Endowment and The California Wellness Foundation. The California Endowment is a private organization dedicated to improving health care coverage and health benefits. The California Wellness Foundation helps California citizens by providing grants for healthcare, health education, and disease prevention.
The federal government eased the strain on recently unemployed workers by providing subsidies for health insurance coverage under COBRA through the American Recovery and Reinvestment Act of 2009 (ARRA). ARRA was signed into law by President Obama. This helps to maintain health insurance coverage for large numbers of unemployed workers. COBRA (Consolidated Omnibus Budget Reconciliation Act of 1985) is a law passed by the US Congress and signed by President Reagan. The law mandates that insurance companies give employees the option to continue health insurance coverage after leaving or losing employment. Despite the infusion of federal assistance through the ARRA, many cannot afford the COBRA health insurance premiums and lose their health insurance anyway. However, the UCLA study concluded the utilization of COBRA by laid-off employees doubled with the addition of the federal ARRA assistance. The federal subsidy to COBRA health insurance coverage made continuing health insurance coverage possible for large numbers of people.
1.1 million children in California did not have health insurance in 2007 rising to 1.5 million children in 2009. The study noted that public coverage expanded to assist children thereby keeping the rate of uninsured children relatively low in comparison to adults. The Medi-Cal and Healthy Families programs provide what the study describes as a “substantial safety net” for children. Approximately one out of every three children in California receive their healthcare benefits through the Healthy Families or Medi-Cal programs. Due to lack of funds, the Healthy families program was forced to freeze enrollment. Governor Schwarzenegger is pushing for the elimination of the Healthy Families program and reducing Medi-Cal benefits to the minimum amount required by federal law. The UCLA study notes that “an immediate increase in federal support for the state’s Medi-Cal and Healthy Families programs would stabilize the health care safety net. In addition, national health care reform could help stabilize health insurance markets in the state and provide more, and more stable, opportunities for Californians to obtain affordable health insurance coverage.”
The American Recovery and Reinvestment Act of 2009 (ARRA), also known as “the Stimulus”, was passed into law by President Barack Obama on February 17, 2009 and provides the federal government with the means to increase federal health insurance subsidies to Californians. The Stimulus cut federal taxes and expands unemployment benefits, education spending, healthcare benefits, energy sector investments, and infrastructure development. The ARRA stimulus is intended to create jobs and promote investment during the current economic recession. This is Keynesian economics which argues that government spending can compensate for a drop in consumer spending during economic downturns. No Republicans in the US House of Representatives voted for the bill. Three Republican Senators voted for the ARRA. Although the bulk of the ARRA focuses on stabilizing and promoting economic development, it also includes limitations on executive compensation in banks receiving federal assistance. The ARRA provides tax cuts and expands healthcare spending. Medicaid received over $85 billion and COBRA received nearly $25 billion. ARRA expands medical care for the military and their families, funds prevention and wellness programs, contributes to the Veterans Health Administration, funds healthcare services on Indian reservations, promotes health related research at the NIH (National Institutes of Health), expands payments for Community Health Centers, and funds the development of health information technology.

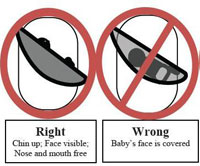
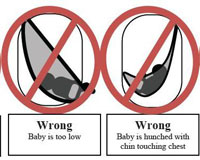
The CPSC (US Consumer Product Safety Commission) has identified 14 deaths associated with sling-style infant carriers. Three infants died in 2009 and 12 deaths involved babies under 4 months of age. The highest risk babies are those with low birth weight, were prematurely born, or babies experiencing breathing difficulties due to issues such as a common cold. The CPSC has issued official directions on the correct way to use a sling carrier such that babies will not suffocate. In addition, the CPSC has recalled 3 baby sling devices due to manufacturing issues.
Infant baby slings present two suffocation dangers. Since babies cannot control their heads in the first few months of life due to weak musculature, the sling can press against the nose and mouth. This can lead to suffocation within one to two minutes. Also, the sling may rest the baby in a curled position with the chin bending toward the chest. In this scenario, airways are restricted and the baby cannot cry for help while slowly suffocating.
The CPSC has issued simple steps to prevent these avoidable tragedies. The CPSC “recommends that parents and caregivers make sure the infant’s face is not covered and is visible at all times to the sling’s wearer. If nursing the baby (is) in a sling, change the baby’s position after feeding so the baby’s head is facing up and is clear of the sling and the mother’s body.” Frequent checking of the baby’s position is urged, especially after nursing.
In related news, the CPSC in cooperation with Ellaroo LLC, has recalled 1,200 units of the Ellaroo Ring Sling baby carrier because the aluminum rings can bend or break. This may cause the fabric to slip through the rings and infants may fall out of the carrier. Manufactured in India, this recall is limited to lot numbers 03/07 and 07/04. The lot number can be found printed on the sling’s label. The CPSC, in cooperation with Infantino LLC, has recalled approximately 100,000 units of the Infantino SlingRider with item numbers: 141-210; 151-210; 151-528; and 151-534. Manufactured in China, Infantino has received 10 reports of the plastic adjustment sliders breaking. In eight cases, the the babies fell out of the carriers due to this issue. The CPSC, in cooperation with Zolowear Inc., announced a voluntary recall of ZoloWear Infant Carriers/Slings. Manufactured in the US, only 165 units were affected in the recall. One report of an issue related to the stitching that attaches the webbing to the carrier/sling prompted the recall. No one was injured and this recall was a preventative measure.
Below is a diagram released by the CPSC displaying the correct method for resting a baby in a baby sling and four incorrect, and potentially dangerous, positions.

Companies may add engineered nanomaterials (materials manipulated at the molecular level) and other GRAS substances into foods without notifying the FDA. Companies may make their own determination of whether or not a food is GRAS and are not required to tell the FDA if the substance exists or how the GRAS determination was made. The GAO notes that substances previously categorized as GRAS have been banned due to health hazards. The GAO report states, “FDA does not know to what extent, or even whether, companies track evolving scientific information about their GRAS substances. FDA's approach to regulating nanotechnology allows engineered nanomaterials to enter the food supply as GRAS substances without FDA's knowledge.” The GAO report also notes that all foods containing engineered nanomaterials are regulated in many countries including Canada and the European Union but not in the U.S. .
Sodium cyclamate, an artificial sweetener, was a GRAS substance banned by the FDA. Sodium cyclamate was discovered by a graduate student at the University of Illinois when he was working on the synthesis of an anti-fever drug. The student’s cigarette came into contact with the cyclamate when he placed it on the lab bench. When he resumed smoking, the graduate student discovered that the cyclamate had a sweet taste from the flavor it imparted to his cigarette. Research connecting sodium cyclamate to cancer and testicular atrophy caused the FDA to pull sodium cyclamate from the market. Although the FDA took action against sodium cyclamate, the GAO asserts that the FDA is unresponsive to most concerns from individuals and consumer groups concerning GRAS substances in the U.S. food supply. In 11 citizen petitions submitted to the FDA between 2004 and 2008, the FDA came to a decision on only one of these investigative complaints.
The GAO recommends that the FDA develop a strategy to identify GRAS ingredients in foods and how companies determine GRAS status for their foods. The GAO also recommends that the FDA develop a strategy for reconsidering GRAS ingredients already present in the food supply and to develop an approach for handling engineered nanomaterials. The FDA agrees with the GAO findings and notes that they are beginning an internal deliberation regarding GRAS substances.
The US House of Representatives has passed the ‘Health Insurance Industry Fair Competition Act’ by a vote of 406 to 19. This legislation repeals antitrust protections enjoyed by the health insurance industry. This is an overwhelming majority vote supporting the repeal of longstanding antitrust exemptions for insurance companies and the bill has over 70 cosponsors. Representing the 5th district of Virginia, Congressman Tom Periello sponsored this bill to end monopoly protections for health insurance companies.
The McCarran-Ferguson Act of 1945 gave insurance companies exemption from antitrust laws allowing them to legally fix prices, collude with one another, and to divide market turf amongst themselves. Spokesmen from several branches of the insurance industry oppose this legislation with many asserting that it will not help to control rising healthcare costs. The Consumer Federation of America (CFA) estimates that this legislation will save Americans approximately $5 billion per year. President Obama favors repealing antitrust protections for insurance companies.
In an interesting twist, the Senate has not stated whether or not it will consider this legislation despite its massive bipartisan support in the House. The ‘Health Insurance Industry Fair Competition Act’ (HR 4626) is only two pages of text in contrast to the enormous healthcare bill recently stalled in the US Senate. It seems the US House of Representatives has passed a simple, clean bill that anyone can read in a matter of minutes. A total of 253 Democrats voted in favor of the legislation joined by 153 Republicans. The only nays came from a small group of 19 Republicans. The act sailed through the House in merely two days providing easy passage. Surprisingly, the passage of this historic act has received muted attention by the press.
Opinion polls consistently point to frustration amongst Americans over the lumbering and inefficient process of passing legislation marred by corruption from lobbyists. Equally unpopular are amendments loaded with pork that weigh down legislation and drive up costs. This act moved swiftly and lacks any costly amendments. As a test to the Federal process, should this bill move through the Senate it would represent a breakthrough of the gridlock that hampers the US government and fosters rewards for special interest groups over US citizens.
The official US House of Representatives long title of this act is “To restore the application of the Federal antitrust laws to the business of health insurance to protect competition and consumers.” The exact text of the bill reads:
(a) Amendment to McCarran-Ferguson Act- Section 3 of the Act of March 9, 1945 (15 U.S.C. 1013), commonly known as the McCarran-Ferguson Act, is amended by adding at the end the following:
(c) Nothing contained in this Act shall modify, impair, or supersede the operation of any of the antitrust laws with respect to the business of health insurance. For purposes of the preceding sentence, the term `antitrust laws' has the meaning given it in subsection (a) of the first section of the Clayton Act, except that such term includes section 5 of the Federal Trade Commission Act to the extent that such section 5 applies to unfair methods of competition.'.
(b) Related Provision- For purposes of section 5 of the Federal Trade Commission Act (15 U.S.C. 45) to the extent such section applies to unfair methods of competition, section 3(c) of the McCarran-Ferguson Act shall apply with respect to the business of health insurance without regard to whether such business is carried on for profit, notwithstanding the definition of `Corporation' contained in section 4 of the Federal Trade Commission Act.
Learn more about healthcare and insurance issues affecting nurses, doctors, and patients at HealthCMI Online, http://www.healthcmi.com .
Wet age related macular degeneration (AMD) is treatable with Lucentis. Wet AMD causes vision loss due to abnormal blood vessel growth in the eye. The blood vessels bleed, leak, and cause scarring therefore severely damaging vision. The FDA has approved Lucentis (ranibizumab) for the treatment of wet AMD but many physicians favor Avastin (bevacizumab) because it is significantly less expensive and yet chemically similar. Both products are made by Genentech, the biotech pharmaceutical division of Roche.
The use of Avastin for AMD is not approved by the FDA. This off-label (non-approved) use of Avastin generates significant controversy. According to Genentech, Lucentis is a superior treatment for AMD. Many doctors favor Avastin because it costs approximately $150 per treatment compared with that of Lucentis at $2,000 per treatment. The Lucentis-Avastin controversy will end in February 2011 with the completion of the National Eye Institutes’s (NEI) clinical trials that compare the two drugs for the treatment of wet AMD. The NEI study evaluates the safety and efficacy of both Lucentis and Avastin for the treatment of AMD.
The final data collection of the study is occurring now even though the final conclusions will be officially released next year. This is a massive study chaired and directed by doctors from the Cleveland Clinic, University of Pennsylvania, and Duke University. Over 55 medical groups from California to Florida participate in this effort. This includes centers such as the Mayo Clinic in Rochester, Minnesota; the Massachusetts Eye & Ear Infirmary in Boston, the Duke University Eye Center in North Carolina, the University of Wisconsin, the West Coast Retina Medical Group in San Francisco, California; the University of California-Davis Medical Center in Sacramento, and the Retina-Vitreous Associates Medical Group in Beverly Hills, California to name a few.
The study will determine whether or not Lucentis is safer and more effective for the treatment of wet AMD over Avastin. The financial stakes are high for the biotech giant Genentech whose profits from Lucentis over Avastin for the treatment of AMD are significant. More importantly, the study will determine what is the best course of treatment for patients looking to save their eyesight. The NEI study results have not been posted yet the information is vital for patients suffering from AMD. Looking forward, will doctors and participants from the clinical trials leak anecdotal information over the course of the next year?
A separate study conducted by researchers from the Boston University School of Medicine and the VA Boston Healthcare System which appears in the American Journal of Ophthalmology concludes that there is no difference between Lucentis and Avastin for the treatment of AMD. This is hardly the final word. This study was only conducted on 20 subjects for a period of six months. At best, this study is inconclusive and on the downside this study may be inadvertently steering doctors away from using the best possible treatment for AMD. As a result of this preliminary investigation, many doctors have already concluded that there is no difference between Avastin and Lucentis. We are a year out from knowing the answer to the Lucentis-Avastin controversy. Look to February 2011 to learn the answer to this very important question that will ultimately help determine the best course of treatment for patients suffering from AMD. Once the NEI study is published in 2011, the pharmaceutical controversy is over and patients will benefit from this knowledge.
Learn more about nursing and medical continuing education news at HealthCMI by clicking Nursing Continuing Education Online.

